


Respiratory tract infection is one of the most common infectious diseases in humans in the world, and the incidence rate ranks first in various infectious diseases. 20% of the deaths of preschool children worldwide are respiratory infections, causing 400 million people to be infected with respiratory pathogens every year. The World Health Organization (WHO) published an article in the "Lancet" magazine in 2005: in 2000-2003, 1060 per year Of the 10,000 children under the age of 5, deaths due to infectious diseases accounted for 54%, has become the main cause of death of children under the age of 5, and is also one of the public health problems of the elderly.
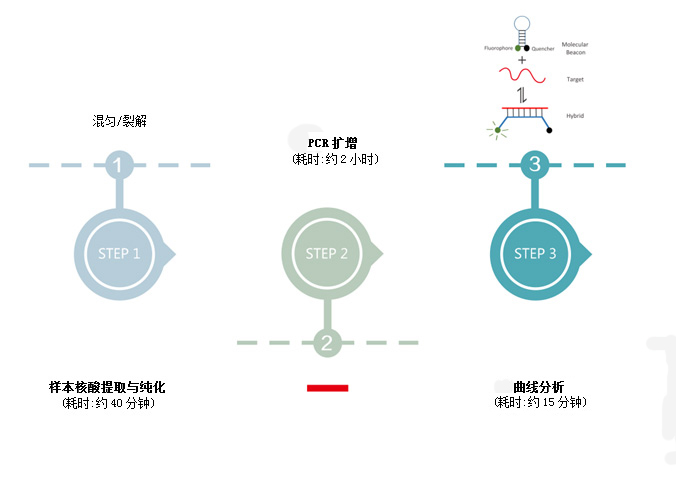
Based on PCR-fluorescent probe method, qualitative detection of RNA and DNA of pathogens in pharyngeal swabs of subjects.
|
Project indicators |
7 immunofluorescence tests |
Single fluorescent PCR |
16 inspections of liquid chips (U.S. luminex) |
Capillary electrophoresis |
Method of this project |
|
Detection of pathogens |
7 inspections |
1 ~ 2 inspection |
16 inspections |
16 inspections |
18 inspections |
|
Detection cycle |
2 hours |
3 hours |
6 hours |
4 hours |
1.5 hours |
|
Sensitivity |
low |
high |
high |
high |
high |
|
Sample flux |
1 |
6 ~ 12 |
96 |
48 |
48 |
|
Experimental operation |
Extremely complex and slow |
Relatively complex |
Relatively complex |
Relatively complex |
Simple and fast |
|
Product contamination |
no |
Not easy to pollute |
Easily contaminated |
Relatively easy to pollute |
Not easy to pollute |
|
Clinical charges |
low |
high |
high |
high |
low |
|
Respiratory pathogen multiple typing detection kit (multiple fluorescent probe melting curve method) |
|
|
Pathogen type |
New coronavirus 2019-nCoV, coronavirus (HCoV-229E, HCoV-OC43, HCoV-NL63, HKU1), influenza A virus, influenza B virus, respiratory syncytial virus, adenovirus, parainfluenza virus Type II, Type III), rhinovirus, human metapneumovirus, Bocavirus, B. pertussis, Chlamydia pneumoniae, Mycoplasma pneumoniae. |
|
Sensitivity |
10 copies / μL |
|
Linear range |
10-1.0x109 copies / μL |
|
Accuracy |
Use national reference products as the control, the coincidence rate of the test results is 100% |
|
Precision |
Coefficients of variation within and between batches CV are less than 4.5% and 3.5%, respectively |
|
Specificity |
100% specificity, no cross-reactivity with other clinically common pathogenic microorganisms |
|
Anti-interference |
Commonly used drugs for the treatment of respiratory diseases do not interfere with this product |
Diagnosis of pathogen classification of respiratory tract patients
Respiratory tract risk assessment
High-risk groups, newborn screening